Navigating Stargardt Disease: Infrared Therapy for Macular Health
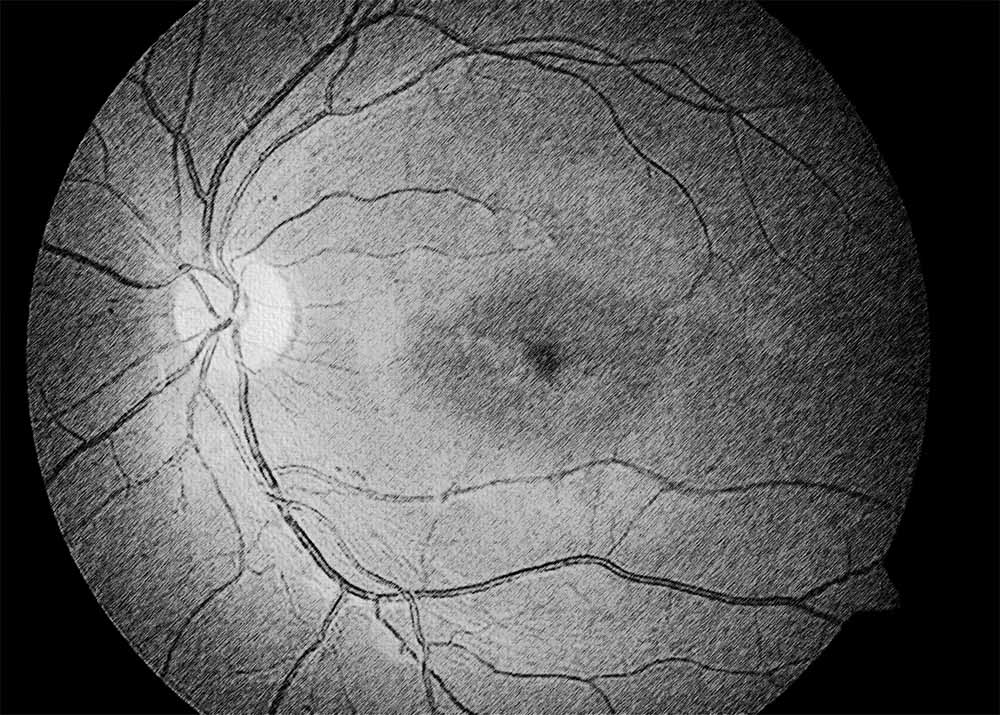
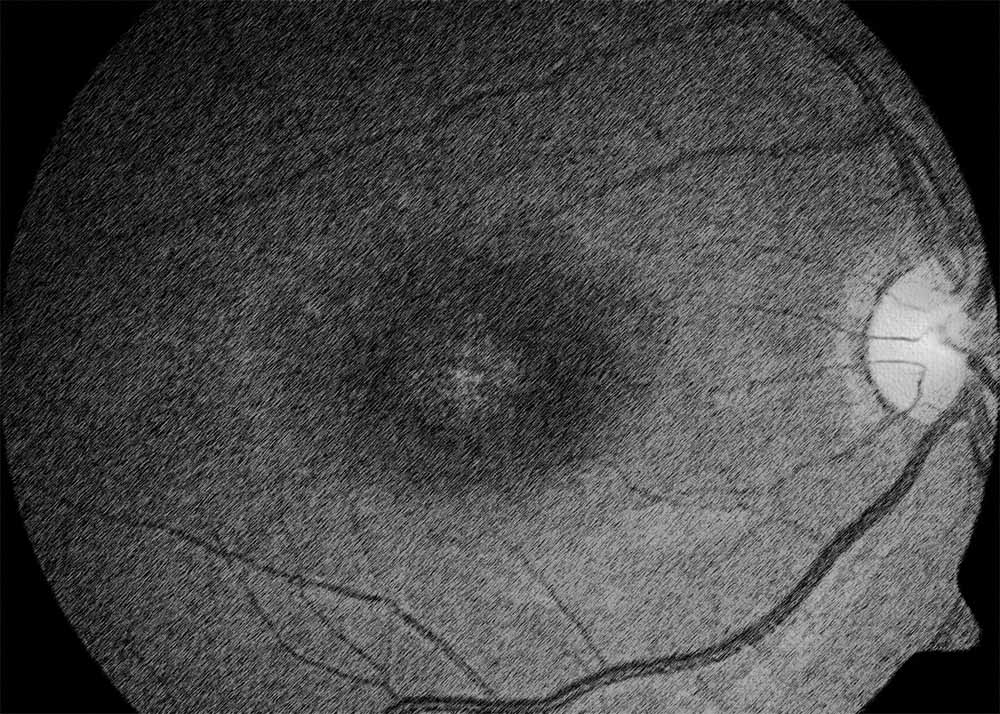
Stargardt disease, also known as Stargardt macular dystrophy or juvenile macular degeneration, is an inherited retinal disorder characterized by progressive central vision loss. It typically manifests in childhood or adolescence and is caused by mutations in the ABCA4 gene, leading to the accumulation of lipofuscin deposits in the retinal pigment epithelium (RPE) and subsequent degeneration of the macula. Diagnosis of Stargardt disease involves a comprehensive eye examination, including visual acuity testing, fundus photography, optical coherence tomography (OCT), and genetic testing to confirm the presence of ABCA4 mutations.
There are several types of Stargardt disease, classified based on the age of onset, clinical presentation, and genetic mutations involved. These include classical Stargardt disease, which typically presents in childhood or adolescence with central vision loss and characteristic macular changes on fundus examination. Late-onset Stargardt disease, also known as adult-onset Stargardt disease or fundus flavimaculatus, may manifest later in adulthood with milder visual symptoms and slower disease progression. Regardless of the subtype, Stargardt disease shares common features of macular degeneration and progressive vision loss.
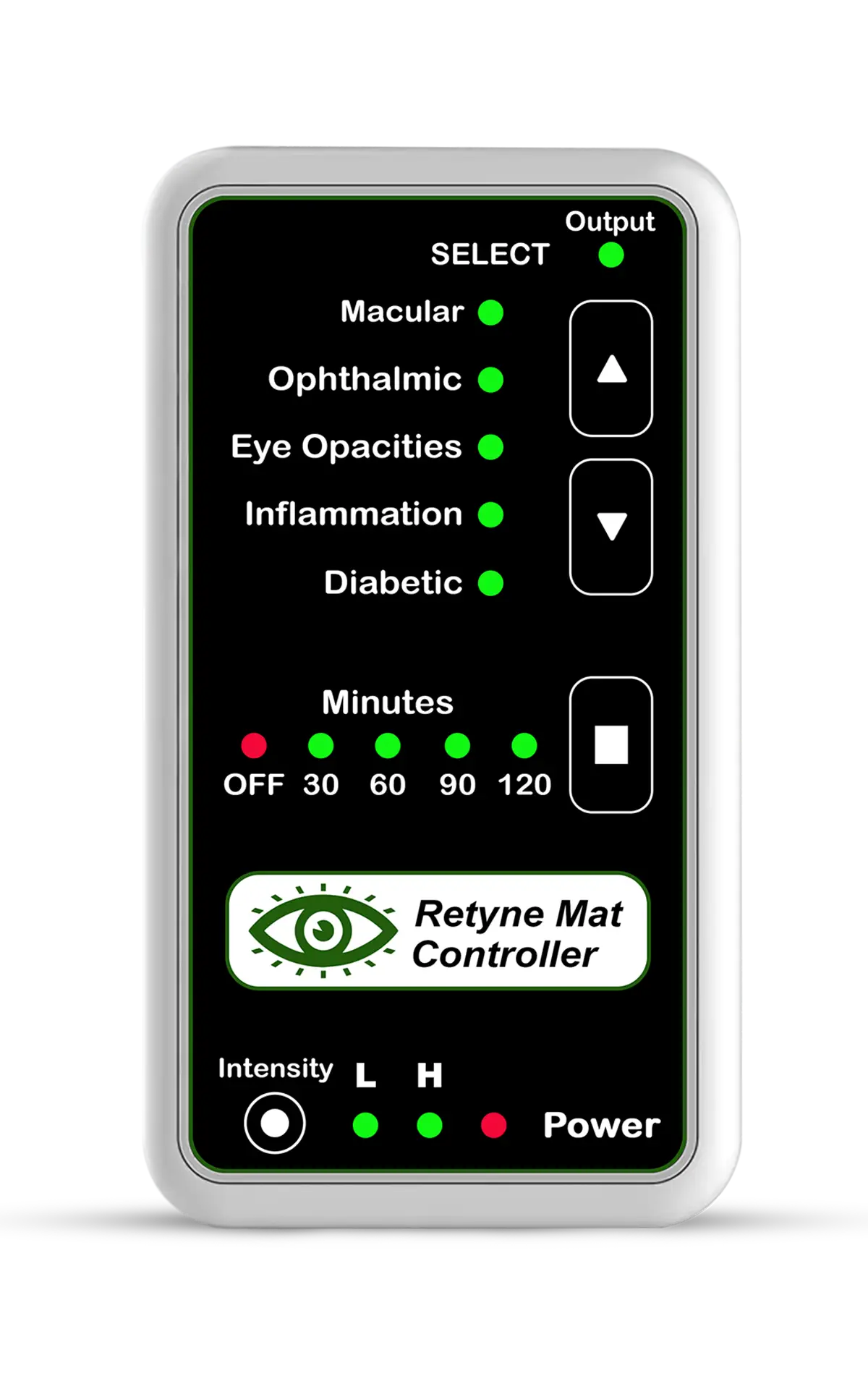
The Retyne Infrared Eye Treatment Mask offers a promising therapeutic approach for managing the symptoms of Stargardt disease, leveraging the benefits of invisible infrared light therapy to target the underlying pathophysiology of the condition. Program #1 on the Retyne controller delivers precisely calibrated infrared light to the affected retinal tissues, aiming to stimulate cellular metabolism, reduce oxidative stress, and promote RPE cell function and survival. By harnessing the therapeutic potential of invisible infrared light, the Retyne mask provides patients with a non-invasive and targeted treatment option for Stargardt disease.
Incorporating the Retyne Infrared Eye Treatment Mask into the management of Stargardt disease offers several potential benefits for patients, including improved visual function, slowed disease progression, and enhanced quality of life. By delivering infrared light therapy directly to the affected retina, the Retyne mask may help reduce the accumulation of lipofuscin deposits, mitigate photoreceptor damage, and preserve central vision. Additionally, the non-invasive nature of infrared light therapy makes it well-tolerated and suitable for long-term use in managing chronic retinal conditions.
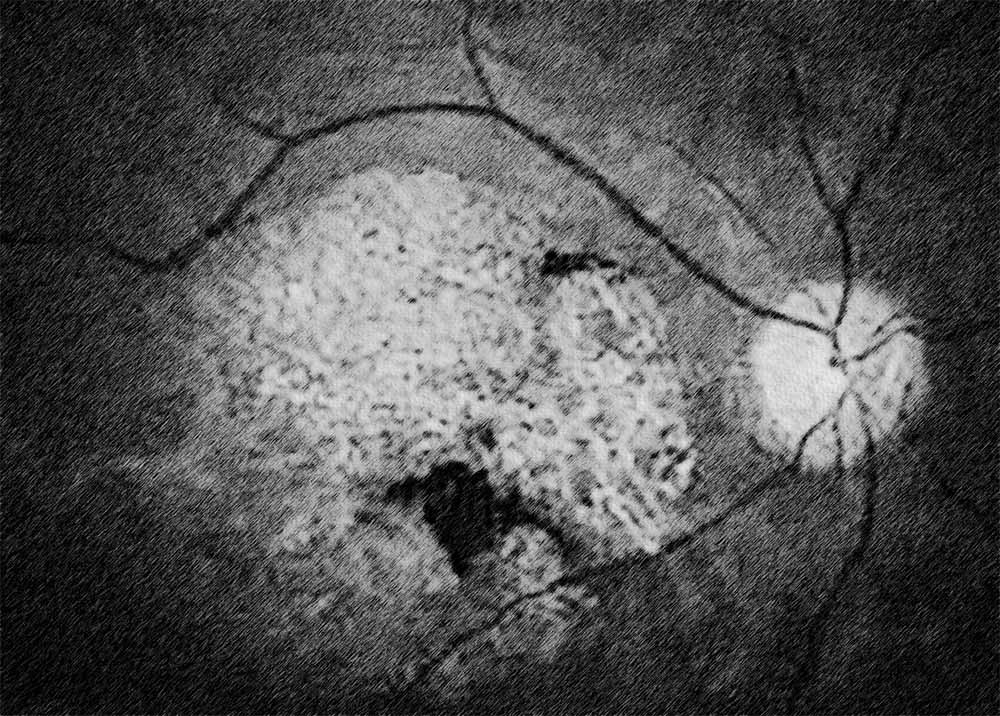
Stargardt disease shares certain similarities with other forms of macular degeneration, particularly in terms of RPE dysfunction and photoreceptor degeneration. As such, treatment modalities that target the underlying mechanisms of macular degeneration, such as infrared light therapy, may also hold promise for managing Stargardt disease. Program #1 on the Retyne controller offers a tailored approach to addressing the specific needs of patients with Stargardt disease, delivering targeted therapy to the affected retina to alleviate symptoms and preserve visual function.
In conclusion, Stargardt disease represents a challenging inherited retinal disorder characterized by progressive central vision loss. The Retyne Infrared Eye Treatment Mask offers a novel therapeutic option for managing the symptoms of Stargardt disease, providing patients with a non-invasive and targeted approach to treatment. By leveraging the therapeutic benefits of invisible infrared light therapy, the Retyne mask holds promise for improving visual outcomes and enhancing the quality of life for individuals with Stargardt disease.
The Retyne eye treatment mask employs a specific array of frequencies (0.15, 0.18, 0.8, 5.5, 33.2, 172.3, 471.2, 557.82, 603.44, 921.88) meticulously tailored to address symptoms associated with Stargardt disease. Each frequency is carefully selected based on its documented effectiveness in managing and alleviating Stargardt disease. Retyne's innovative approach involves the transformation of these frequencies into invisible infrared light output, heralding a pioneering fusion of frequencies with light—a revolutionary technology pioneered by Retyne Labs.
Drawing inspiration from the pioneering research of Dr. Rife, who unearthed the therapeutic potential of precise frequencies and harnessed light for their propagation, Retyne's methodology embraces contemporary insights into invisible infrared technology. By leveraging current advancements and building upon historical investigations into frequency-based light transmission, Retyne has developed the innovative Retyne Eye Treatment Mask. This cutting-edge device represents the synthesis of modern breakthroughs in visual healthcare, offering a comprehensive solution rooted in both tradition and progress.
Stargardt disease General Group exists at program 2067 on the International ETDFL frequency list (0.15, 0.18, 0.8, 5.5, 33.2, 172.3, 471.2, 557.82, 603.44, 921.88)
Compatibility
Standalone controller (Program #1) (Controller shipped with Retyne Eye Treatment Mask)
RDPV4 (Direct connect, use group 2067)
RDPV4 Light Mask Program button 1

Click here for instructions on using the Retyne Mask + Controller