Managing Corneal Transplant Rejection with Retyne Infrared Therapy
Corneal transplant rejection is a significant concern following corneal transplantation surgery, where the recipient's immune system attacks the donor cornea, leading to graft failure. There are two main types of corneal transplant rejection: hyperacute rejection, occurring within minutes to hours after surgery, and acute or chronic rejection, which can manifest weeks, months, or even years later. Symptoms may include redness, pain, decreased vision, and light sensitivity. Diagnosis typically involves clinical examination, including slit-lamp biomicroscopy and corneal imaging, along with monitoring for signs of rejection such as corneal haze, edema, and vascularization.
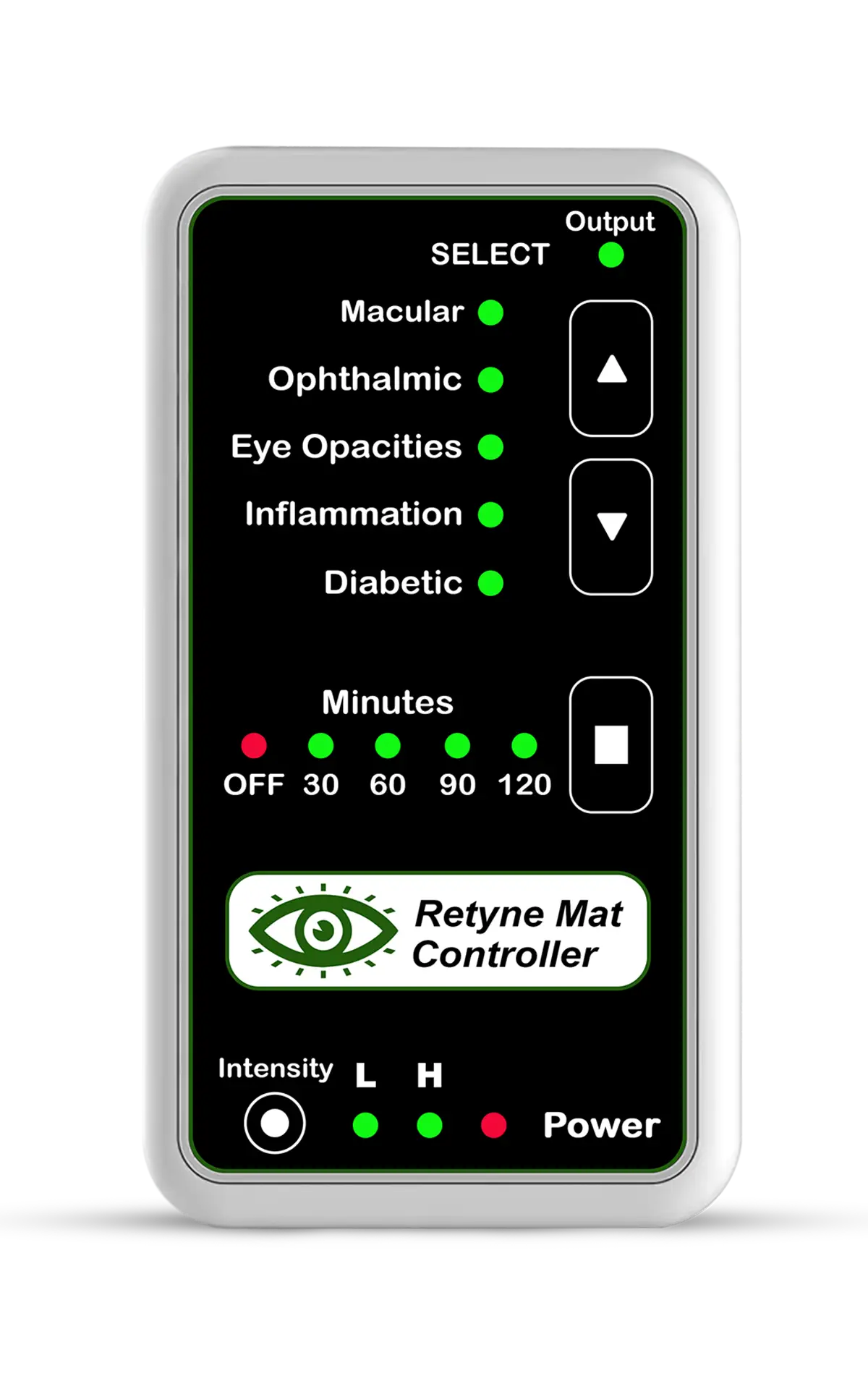
The Retyne Infrared Eye treatment mask presents a promising approach to managing the symptoms of corneal transplant rejection, particularly in cases of acute or chronic rejection. Program #2 on the Retyne controller delivers therapeutic infrared light directly to the cornea, where it penetrates deep into the tissue, promoting cellular repair and reducing inflammation. By modulating the immune response and enhancing tissue healing, infrared therapy can help alleviate symptoms associated with corneal transplant rejection and support graft survival.
In addition to its therapeutic effects, the Retyne mask offers a non-invasive and convenient treatment option for individuals experiencing corneal transplant rejection. Patients can easily incorporate infrared therapy into their post-operative care regimen, undergoing treatment in the comfort of their own homes. Regular use of the Retyne mask as part of a comprehensive treatment plan can help mitigate the risk of rejection episodes and improve long-term graft outcomes, ultimately enhancing visual function and quality of life for transplant recipients.
Furthermore, the Retyne Infrared Eye treatment mask provides symptomatic relief from common manifestations of corneal transplant rejection, such as pain, redness, and discomfort. The invisible infrared light emitted by the mask penetrates deep into the corneal tissue, soothing inflammation and promoting healing. By reducing the inflammatory response and enhancing tissue integrity, infrared therapy can help alleviate the symptoms of rejection and improve patient comfort during the recovery process.
Overall, the Retyne mask represents a valuable adjunctive therapy for managing corneal transplant rejection, offering both therapeutic and symptomatic relief through the utilization of innovative infrared light technology. By harnessing the power of infrared therapy, individuals undergoing corneal transplantation can optimize graft survival, minimize the risk of rejection, and achieve improved visual outcomes. As part of a comprehensive treatment approach, infrared therapy with the Retyne mask has the potential to revolutionize post-operative care for corneal transplant recipients, paving the way for enhanced recovery and long-term graft success.
The Retyne eye treatment mask utilizes a general selection of frequencies (0.07, 0.12, 0.6, 0.87, 2.25, 22.5, 187.5, 396.5, 587.5, 696.5) specifically tailored to address Corneal Transplant Rejection. These frequencies have been selected for their proven effectiveness in managing and treating this visual condition. Retyne's approach involves converting each frequency into invisible infrared light output, marking a groundbreaking fusion of frequencies with light—a pioneering technology pioneered by Retyne Labs.
Inspired by the groundbreaking work of Dr. Rife, who identified healing properties in specific frequencies and utilized light for their transmission, Retyne's innovative method capitalizes on current research on invisible infrared technology and builds upon past studies on light transmission through frequency sources. The result is the Retyne eye Treatment Mask, a convergence of state-of-the-art advancements in the field of visual care.
The general set group exists at program 811: Corneal Transplant Rejection: 0.07, 0.32, 0.95, 7.5, 84, 193.93, 237.5, 487.5, 706.21, 946.5
Compatibility
Standalone controller (Program #2) (Controller shipped with Retyne Eye Treatment Mask)
RDPV4 (Direct connect, use group 811)
RDPV4 Light Mask Program button 2

Click here for instructions on using the Retyne Mask + Controller